Dissolved oxygen in lake habitats
Dissolved oxygen is essential for aquatic habitats. But what is it, and how does it get there? When oxygen enters water, it changes form and becomes dissolved oxygen (DO). DO is non-compound oxygen, or oxygen that is not bonded to any other element.
Oxygen enters the water in a few ways: First, it is naturally generated by phytoplankton, algae, seaweed and other aquatic plants as they photosynthesize. It can also be mixed into a lake by through aeration, like wind blowing across the water’s surface.
The aeration of water can be caused by wind (creating waves), rapids, waterfalls, ground water discharge or other forms of running water. Man-made causes of aeration vary from an aquarium air pump to a hand-turned waterwheel to a large dam.
After DO enters the water, fish can draw it in through their gills, and aquatic plants can draw it up through their roots and release it into the air and water.
How much dissolved oxygen do fish need?
Although all forms of aquatic life need DO, not all need the same amount. Fish like largemouth bass, longear sunfish and bluegill all require specific levels of DO and the right temperatures to survive. Bottom dwellers like aquatic worms and bacteria are less picky. They can live with almost no oxygen, and some bacteria are specialized to live without any.
In general, the following amounts of DO are necessary for life:
Bottom feeders, crabs, oysters and worms need minimal amounts of oxygen (1-6 mg/L), while shallow water fish need higher levels (4-15 mg/L).
Dissolved oxygen cannot reach the very bottom of the lake. That is why fish can only live in limited portions of the water — and that portion depends on the lake’s DO content. The Lilly Center research team takes measures for DO during summer lake sampling, conducted on 14 local lakes from June-September every year! In 2022, here are the averages of what we found:
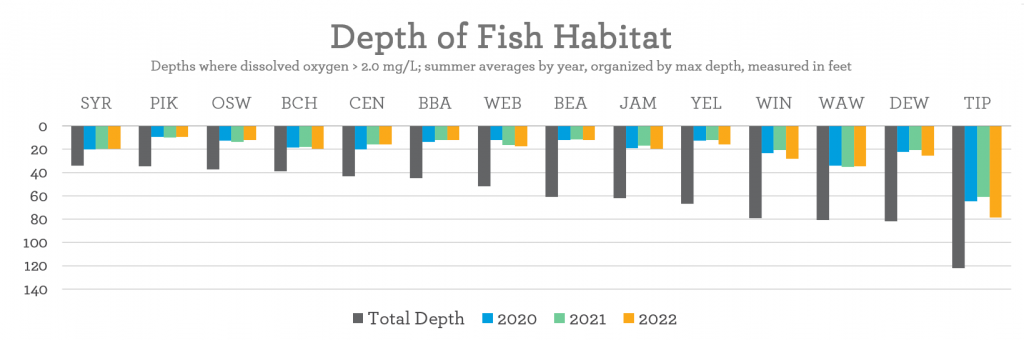
Did you know that by reducing the amount of material decomposing at the bottom of a lake, we can slow that use of oxygen and make more room in the lake for fish to inhabit in the summer? Plants are an important part of a lake’s ecosystem, but too many of them can result in too little DO for other aquatic life.
What affects dissolved oxygen levels in water?
Aquatic and non-aquatic plants are important to the overall health of a lake. Shorelines (especially when anchored by native plant species) serve as the gatekeeper, determining what enters the water. Aquatic plants provide habitats for underwater life and help cycle fresh DO into the water.
If a body of water has high concentrations of certain nutrients, however, DO levels are quickly boosted. This is often the result of a robust algae bloom or excessive weed growth. After the algae or weeds die, quite a bit of DO is used for the plant’s decomposition. In some cases, not enough DO is left for fish and other organisms. That is one reason the presence of too many nutrients cause problems on our lakes! On the other hand, the right amount of healthy plants will release the right amount of dissolved oxygen into the water.
How can I help my lake?
There are lots of ways to help protect the health of your lake and its inhabitats, and many of them begin right on your property — whether or not you live on a shoreline! Visit our FAQ to start learning. For our more advanced citizen lake scientists, read through Beneath the Surface, an annual publication that takes a close look at the data we gather.
Want to learn more about dissolved oxygen? Check out these expert sources: